Cathodic protection in the form of sacrificial anodes (Sacrificial Anode Cathodic Protection or SACP) or Impressed Current Cathodic Protection (ICCP) is used to try to reduce the destructive effects of corrosion on ships, boats and marine structures.
This article and Part 2 which will follow, discuss an alternative, simpler, more effective and more economical way to accomplish full protection of ships and marine structures from corrosion.
What is cathodic protection exactly?
The sea is a highly corrosive environment for steel and other material used in the construction of ships and offshore or immersed structures. If not protected, these materials can rapidly waste away due to the corrosion.

A major source of corrosion in ships and marine structures comes from chemically generated electrical currents flowing between parts of the ship or structure. Water, unless it is distilled, conducts electricity. Saltwater is an excellent conductor. In an electric circuit, electrons flow from an anode (negative terminal) to a cathode (positive terminal). Oxidation or rust will occur at the anode, wasting it away.

Cathodic protection works on the basis that the ship’s hull and underwater parts can be forced to be the cathode in the circuit, thus preventing or reducing corrosion. One way this is achieved is by attaching sacrificial anodes of a reactive material to the hull which will then corrode and waste away instead of the hull doing so. Another way of making sure the hull is the cathode and not the anode in the circuit, is to attach non-consumable anodes to the hull which are connected to a DC electrical source inside the ship or structure.

Sacrificial anodes are blocks of metal, such as zinc, aluminum, magnesium among others, attached to the steel or other substrate which are more electrically active than the steel and therefore corrode instead of the ship’s hull and running gear doing so. This approach tends to be used for smaller, lower budget applications.
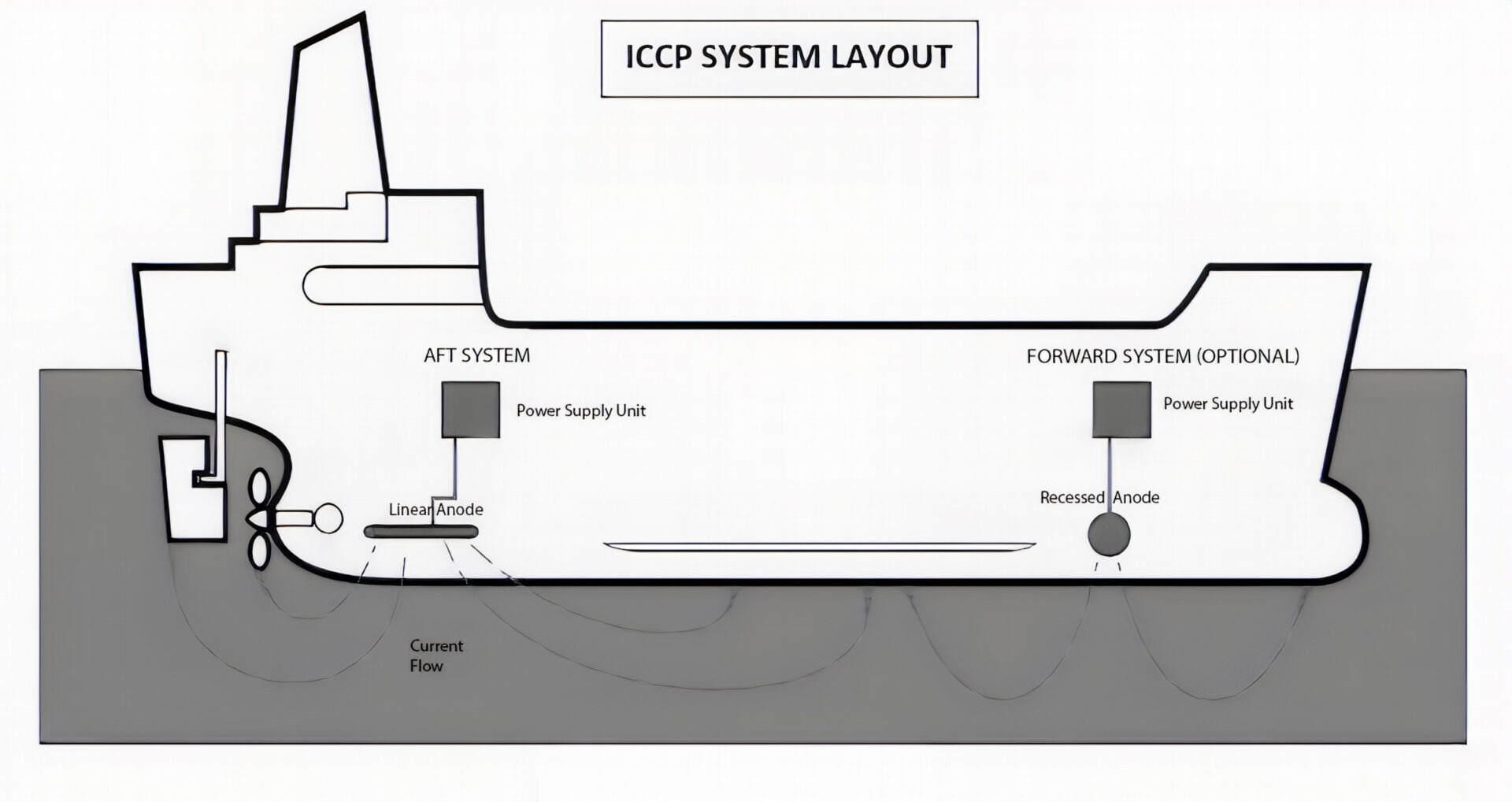
ICCP is generally more common on large ship hulls and large structures. SACP and ICCP can and often are, combined in a hybrid cathodic protection system.

History of cathodic protection
The use of cathodic protection dates back to 1824 when Sir Humphrey Davy working for Britain’s Royal Navy proposed the idea of using iron anodes to protect the copper sheathing of Royal Navy vessels. It did slow the corrosion of the copper, but also decreased its antifouling properties. It was abandoned.
In 1834 Davy’s pupil, Michael Faraday, measured the connection between corrosion weight loss and electric current.
But it was not until around 1930 that cathodic protection began to be used more widely, originally in steel pipelines.
In the 1950s, in an attempt to provide more efficient protection for larger hulls and structures and to reduce the recurring expense, labor and time required to replace sacrificial anodes, ICCP was developed. Another factor that led to this development was the negative impact on the ship’s hydrodynamics when large numbers of sacrificial anodes are used.
This is really as far as this method of trying to prevent the impact of corrosion on ships and marine structures has reached.
Corrosion and protective coatings
Cathodic protection becomes necessary when the protective coating is not protective enough. If the coating is relatively porous to begin with, is damaged by bumps and scrapes, ice, chemicals or cavitation or simply fails and delaminates, then the idea is that the cathodic protection will come to the rescue and prevent disintegration.
In fact, if the steel or other material of the ship’s hull or the immersed structure is thoroughly insulated with a permanent, impermeable, non-conducting coating, then it will not corrode even though no cathodic protection is employed.
What kind of coating would this require?
This coating would have to be completely non-porous, have superior adhesion, and remain intact for the life of the ship or structure. It would have to be tough enough for marine fouling not to be able to penetrate the coating and expose the steel. It would also need to be chemical corrosion resistant and highly resistant to abrasion. The coating would also have to be resistant to cavitation which easily destroys weaker coatings. If the coating suffers mechanical damage, then its adhesion would need to be sufficient to prevent undercreep where local corrosion spreads further under the coating. Any mechanical damage would need to be easily repaired. It would need to be cleanable in the water even with aggressive cleaning methods without suffering any damage.

The problem with conventional hull coatings, including epoxies, anti-corrosive schemes, self-polishing co-polymers, silicone-based foul release coatings and in fact all the conventional coatings in use for general ship protection, is that they do not meet the above requirements. They are generally porous to some degree even when first applied; they are usually soft and easily damaged or removed by cavitation, abrasion or chemicals. They therefore fail in their primary duty which is to protect the material they are coating from corrosion. With these coatings, the use of cathodic protection is a vital requirement.
But cathodic protection brings with it additional expense and hassle: sacrificial anodes are not cheap, and they need to be installed and replaced; plus, they have an adverse effect on the ship’s or boat’s hydrodynamics. ICCP systems are expensive and can be complicated to install, and maintenance heavy. There is a negative impact on the environment where sacrificial anodes are used as they pour excessive amounts of zinc, aluminum or other elements into the water column and sediment as they corrode away.
In general, shipowners and managers seem to be unanimous on the idea that not having to have cathodic protection on their vessels and other structures would be a considerable benefit.

The good news
There is a family of coatings that have been proven to provide all the protection required for steel, aluminum and GRP without the need for cathodic protection in the form of SACP or ICCP.
In Part 2 of this article, in the next edition of Subsea magazine we will go into this in detail, tell you about the coatings, how and why they work, with examples, class endorsement and case studies.
RIP cathodic protection. You have been of huge benefit in protection of steel at sea. Your services are no longer required.
Key takeaways from Part 1
- Corrosion is Public Enemy No. 1 for ships and maritime structures.
- Cathodic protection has been in use for over 200 years to help reduce the harmful impact of corrosion in ships and marine structures.
- This is accomplished using sacrificial anodes and/or Impressed Current Cathodic Protection to reduce the electrochemical impacts that come from having steel and other material in continual contact with water, especially salt water.
- Cathodic protection is an attempt to keep ships’ hulls and other structures from wasting away if and when the protective coating fails or is damaged or for some reason no longer protects the material it is supposed to protect.
- If coatings existed which were able to completely insulate and isolate the steel or other substrate from the water and other corrosive elements, cathodic protection would not be needed.
The good news is that such coatings exist. They are described in Part 2 of this article which can be found here.
Share this post
Contact us for information or a quote for an upcoming project
Do you have a newbuild or docking coming up that you feel one of our coatings would be the perfect fit for? Contact us now and we will answer your questions and provide a quote.








